
COVID-19 Standard Operating Procedure
COVID-19 Standard Operating Procedure for NHS staff now implemented as NHS England release guidance.

COVID-19 Standard Operating Procedure for NHS staff now implemented as NHS England release guidance.
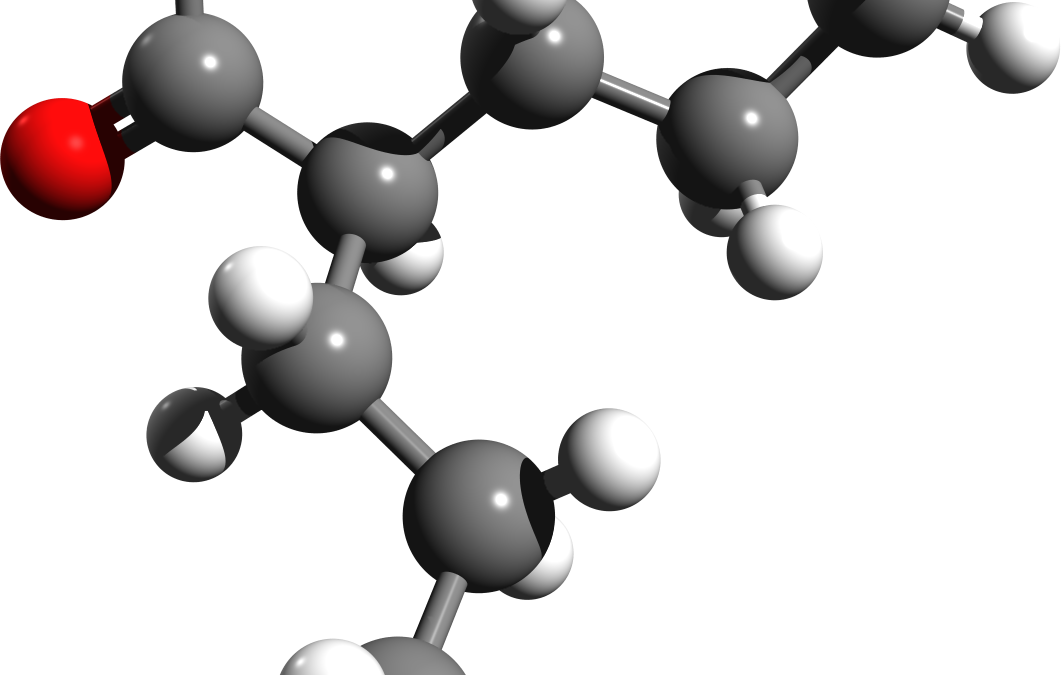
Sodium Valproate guidance – The Sunday Times published in Aril that there are investigations into the prescribing of sodium valporate to pregnant woman.

Serious Shortage Protocols for Hormone Replacement Therapy medicines, issued by The Department of Health and Social Care.
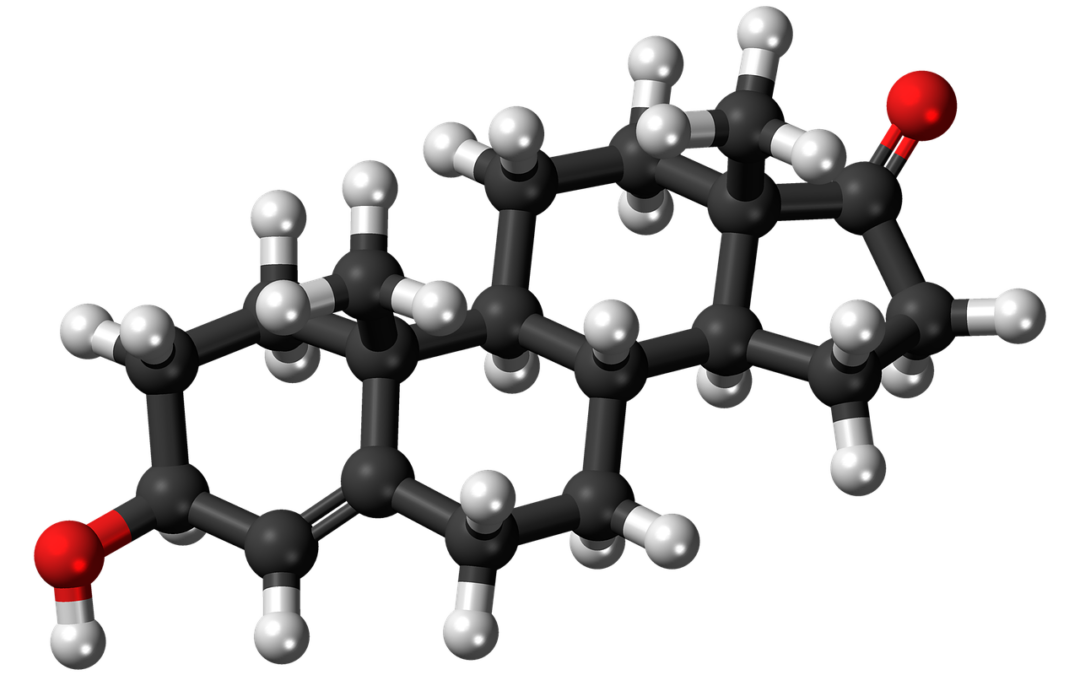
Topical Steroid Withdrawal (TSW) has become a sensational topic on the internet and other forms of media. TSW is a side effect of topical corticosteroid use. Topical corticosteroids are used to treat eczema, psoriasis and contact dermatitis.
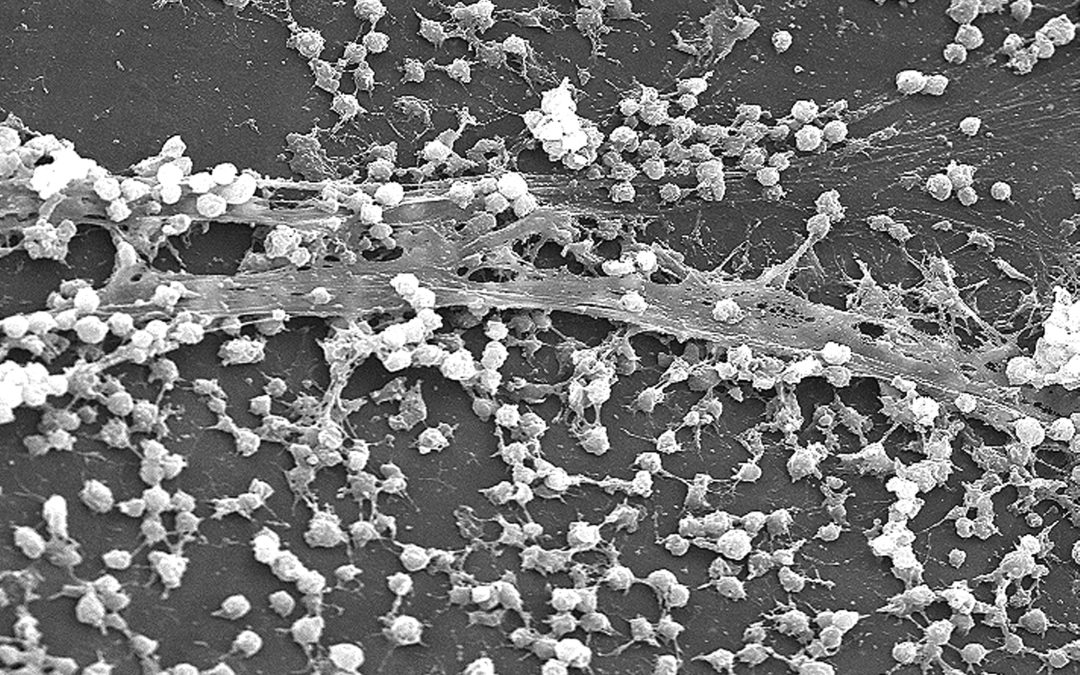
Biofilm is a thin but robust community of bacteria, and sometimes other microorganisms. It is made up of a gel-like material known as extracellular polymeric substances, known as EPS for short.