Academy Training
We can offer a wide range of courses.
Latest News
The Windsor Framework Part 2
After the implementation of the Windsor Framework, the Medicines and Healthcare products Regulatory Agency (MHRA) will license all medicines across the whole of the UK’.
The Windsor Framework Part 1 (recap)
From the 1st of January 2025 the supply of medicines to Northern Ireland will be governed by the Windsor Framework, which will replace the Northern Ireland Protocol.
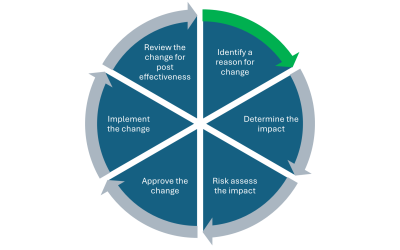
Management of Change in your business
Management of change in your business should be a main cornerstone of any robust and functioning Quality Management System (QMS). The main advantages of having a strong change control process within your business include: