Academy Training
We can offer a wide range of courses.
Latest News
The General Pharmaceutical Council to Reinstate full CPD revalidation
The Government recently announced that the emergency powers that were put in place by the Secretary of State for Health and Social Care to help with the response to the COVID-19 pandemic, will be removed later this year.
Medical Devices – Consultation on the future regulation of medical devices in the United Kingdom
Following the UK’s exit from the European Union (EU), the government identified a unique opportunity to improve how medical devices and in vitro diagnostic medical devices (IVDs) are regulated in the United Kingdom.
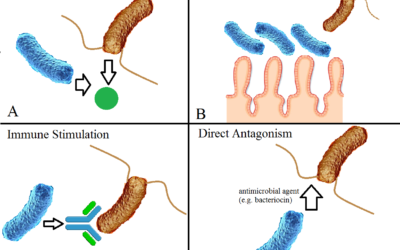
Probiotics and mental health
Probiotics are live bacteria that can colonise the gastrointestinal tract. Probiotic bacteria are often called ‘healthy bacteria’.