The new GDP guidelines on distributing medicines which are the regulations you must adhere to as a wholesale distributor use the word RISK 27 times. As a wholesale distributor you must access all risks within your business. The easiest way to complete this task is to use a risk assessment. Not only does this give you a process to follow to ensure each risk is accessed in the same way but you are also able to provide evidence via documented risk assessments and a register upon request.
Simple in theory, harder in reality. All risks should be based on assessing two elements, severity vs likelihood. Likelihood is based upon how likely the risk is going to occur ranging from highly unlikely through to highly likely. Severity assesses how severe are the consequences if the risk occurs ranging from no detrimental effect to major injury or death.
The three most commonly used risk characterisation systems are:
Colour: Red – Amber – Green
Risks that are assessed as red are for the risks that are unacceptable that will require control mechanisms to be put into place immediately to prevent the risk occurring. A red risk could relate primarily to the severity or likelihood. However, more commonly it is due to correlation between severity and likelihood which results in a red risk rating. Green risks are generally deemed acceptable. Amber risks are in between. Risks that will require attention but red risks will need to be addressed first.
To put it into a visual context take the developments in the safety of cars, first it was seat belts, then car seats, now we have air bags that literally result in you as the driver being placed in a bubble in the result of a crash. First generation cars had little to no protection = red rating, Car with seat belts and car seats = amber rating, bubble car = amber to green rating. The element of risk will always be present.
The colour model is very simple but can result in very generic results as it only gives very broad characterisations of risk. You may have an amber risk that once you have implemented controls to minimise the risk, may still be characterised as amber risk.
Low – Medium – High
This system is very akin to the colour system. Low = green rating high = red rating. This system like the colour system is limited. Generic results could occur and risks can only fit inside these three characterisations resulting on a range of risks being classified as the same risk rating when the reality is that the risks should have further separation.
Hence the creation of a number based system adopted by many which provides for a higher level of characterisation.
Number
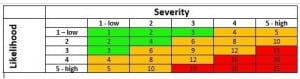
The number system incorporates the colour and low-med-high models. Likelihood and severity are assessed on a scale from one to five, one being low risk and five being high risk. The rating for each element is then multiplied together to give an overall risk rating. These are then categorised into colour model. Red for unacceptable through to green for acceptable. See the table below as a representation.
As you can see by combining the simpler systems and incorporating a number system you have access to a larger range of risk ratings so the risk is more clearly categorised. For example you may have 10 red or high risk ratings, result of which all need addressing at the same time. However, by using a number based approach you may have 9 risks with a 15/16 rating and only one with a 25 rating and therefore this risk should be controlled first.
Do you need help risk assessing your business? Please contact us for help or more information.