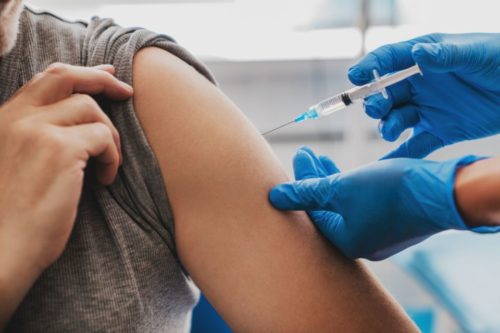
Remember that even after you have been vaccinated you are not exempt from self-isolating.
Remember that even after you have been vaccinated you are not exempt from self-isolating…

Remember that even after you have been vaccinated you are not exempt from self-isolating…

We have heard a few times before that the Healthcare Regulators should…

The Human Medicines Regulations is the main medicines law in the UK and they…

Companies who hold a wholesale licence must understand the…
Five Known Advantages of Blister Packs over bulk medicine bottles. product integrity…