Management of change in your business should be a main cornerstone of any robust and functioning Quality Management System (QMS). The main advantages of having a strong change control process within your business include:
- Management of risk, to both your business, the patient and your products
- Continuity of services
- Business or stakeholder engagement and feedback
Change Management when performed correctly can add value to your processes and prevent unexpected outcomes.
Wholesalers should be proactively documenting and controlling changes to processes, premises, equipment, systems, key personnel and any changes to licenced activity. The way wholesalers manage change can be a key indication of overall quality culture and understanding within a business so it may come as no surprise that it is the first section wholesalers need to complete in their MHRA pre-inspection GDP compliance assessment declaration: https://www.gov.uk/guidance/good-manufacturing-practice-and-good-distribution-practice#types-of-inspection Effective Change Management is a cycle starting with identifying the reason for a change which could come from multiple sources.
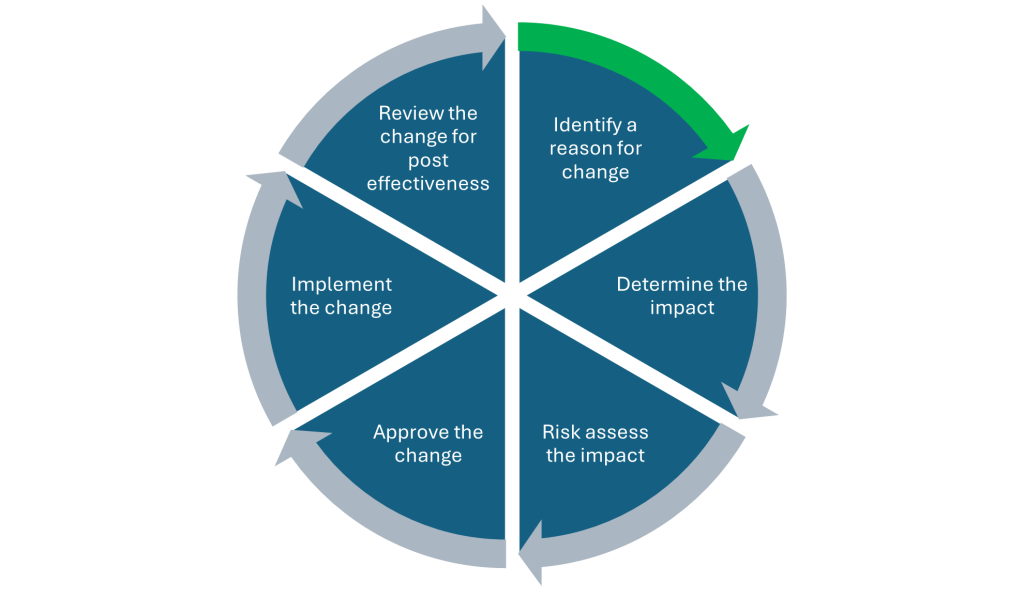
Sources of change could be:
- A process is being created or amended
- A system is being developed or updated
- Equipment is being qualified, updated or its settings or function is being changed
- A company is moving premises, changing the layout or design of existing premises or changing the operational activities performed within the premises
- Key people like the senior management or the Responsible Person are changing
- The business activities are changing such as, but not limited to, adding cold chain to an existing licence, a new third party storage location, trading in unlicensed medicines
- Business acquisitions and mergers which could impact any and all of the above
Once a change has been identified, the next step is to understand the impact of the change and any associated risks. This should be a collaborative process with all key stakeholders or business units impacted by the change. ICH Q9 Quality Risk Management (QRM) should be utilized when assessing changes within GDP organisations: https://www.ema.europa.eu/en/ich-q9-quality-risk-management-scientific-guideline
There are several QRM tools available to wholesalers and the complexity and size of the risk assessment should be commensurate with the complexity of your business.
Once the risks have been determined, ensuring that the necessary actions or steps are performed during implementation should then flow easily. Here at PCL, we understand the importance and value of effective change management and our team of Quality Assurance specialists and Responsible Persons can support your business in managing change to ensure you remain in compliance with your license and you are driving a risk-based approach and continual improvement.
If you would like to know more please contact us.
