Blog
What are your pharmacovigilance hot topics?
The GPvP inspectorate is planning the Good Pharmacovigilance Practice Symposium 2022 and they are keen for help in shaping the event. Read a bit more about it here What topics and themes would you be interested in ?? The MHRA have a survey in which they would be...
RPi (MHRA) beginner’s point of view and reason.
The UK has now got out of the EU (BREXIT complete). As a WDA(H) you will need to transition from RP to RPi as advised by the MHRA.
Pharmacy performance in the community!
Avicena a venture capitalist group has been busy seeing the acquisition of Dudley Taylor Pharmacy group (57 branches across England and Wales) and Shepherds pharmacy group
Pregabalin has been removed from NI Formulary
Pregabalin has been removed from the Northern Ireland (NI) Formulary for neuropathic pain due to an increased number of deaths.
The effects of alcohol on brain health
A recent study suggests that three periods of dynamic brain changes that maybe particularly sensitive to the nuerotoxic effects of alcohol…
Application of Change and Risk Management through Covid and Brexit Challenges
The last four years for pharmaceutical wholesalers have been tough, uncertain demand and outcomes, bumpy supply chains, uncertain of the changes in regulations ahead, staff shortages have added to the risks already in the business model.
The art of auditing can be taught
People often tell us that they cannot audit when they come on our course. This is not true they must just look at things differently. They must do their homework to ensure that they know what they are auditing and why.
Upcoming Public Consultation
Another Medical Device update. The MHRA will shortly be opening a public consultation on the future direction of Medical Device regulation, it is vital that all relevant voices are heard so please monitor the MHRA notices and respond when appropriate.
Deadline Approaching – Medical Device Registration
Deadline approaching – Medical Device Registration for registering Class IIa and Class IIb (other than implants) products with the MHRA is the end of August 2021.
Children, Covid 19 and Safeguarding
In all the noise over the past year regarding Covid 19, children have been affected the most, they may have been the least affected by the virus itself but they have suffered disproportionately from lockdown.
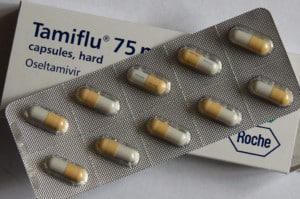
Tamiflu in 2009 and Remdesivir in 2019 and what will be the next wonder drug?
What have we learned in ten years? I was a pharmacist in retail practice in 2009 and was a site that collected all the unused Tamiflu which ended up being destroyed having cost billions across the world.
Digital Consultations, Que busting and Inequality
The number of digital consultations has exploded during the pandemic and for some it has been fantastic, but for other is in not a suitable mode of to name a few; elderly who are not silver surfers and autistic children.