Blog
GDP Training
Essential training for all warehouse staff – our next online GDP training will be on 28th April 2021
New “POM” to “P” Switch announced by the MHRA for Allevia 120mg
Good news for hay-fever sufferers they now have the choice of another over the counter medication which can be bought without a prescription.
Be careful be kind and be safe!
Be careful be kind and be safe! As Easter approaches spare a thought for those who have had their holidays cancelled to care for us.
CBD Oils approved by Novel Foods
Prescribers must ensure they are familiar with the legal and ethical…
Pharmacist beware if you sell Cannabidiol (CBD) oils
New regulations on selling Cannabidiol, CBD, will be in force by the end of…
Health and Social Care White Paper published 11 February 2021
The new white papers main theme is, working together, promoting collaborative partnerships and integration to strip out needless…
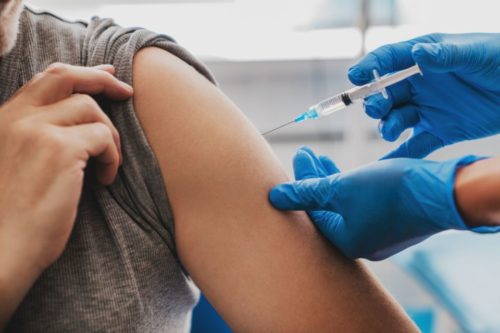
Remember that even after you have been vaccinated you are not exempt from self-isolating.
Remember that even after you have been vaccinated you are not exempt from self-isolating…
Merging of the UK Healthcare Regulators?
We have heard a few times before that the Healthcare Regulators should…
Keep up with changes in UK Medicines Laws and the requirements of Contract, Licence and Authorisation holders.
The Human Medicines Regulations is the main medicines law in the UK and they…
The Importance of Quality
The role, Quality Assurance (QA), is quite literally as described on the tin….
Are we sleep-walking into a Healthcare Crisis in Northern Ireland?
There is much discussion at all levels at the moment about the Withdrawal…
Know your Supplier!
Companies who hold a wholesale licence must understand the…