
by John Finey | Sep 1, 2022 | Blog, Brexit
Do you supply to customers in Northern Ireland – If you do, then you will be aware of a range of issues following Brexit and that we are still some way away from a long term agreement. However, we wanted to highlight a specific issue.
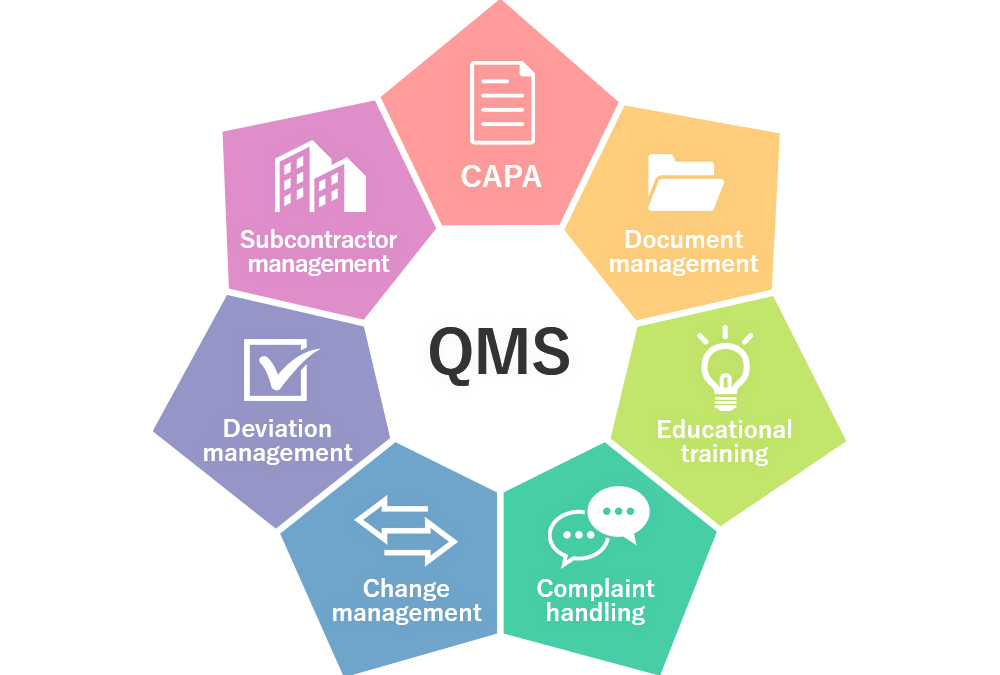
by Elisa Ball | Sep 1, 2022 | Blog, Quality Systems, WDA(H)
Robust Quality Management Systems (QMS) are the heart of our Quality Management. With the right process in place for your business model and the right people managing it, the Quality System will be robust, compliant, easy to navigate and a fantastic tool for trending.

by Kimberley Peck | Sep 1, 2022 | Blog, NHS
In an unprecedented move NHS bosses appeal to the Government to address rising costs before the winter causes a public health emergency.

by Kimberley Peck | Sep 1, 2022 | Blog, MHRA
Following the MHRA and Commitees of Advertising Practice (CAP) releasing a joint enforcement notice regarding advertising Kenalog injections for an unlicenced use, are you as a wholesaler or end user such as clinic aware of the rules around advertising medicines?

by Elisa Ball | Aug 25, 2022 | Blog, Pharmacy
HRT without a prescription, from September for the first time ever, the Medicines and Healthcare Products Regulatory Agency (MHRA) have announced that postmenopausal women in the UK can access a low dose hormone replacement therapy (HRT) product without a prescription, this is a huge step forward in making HRT available safely to women suffering sometimes debilitating symptoms while trying to lead a normal life.

by Thomas Currie | Aug 25, 2022 | Blog
Drones deliver cancer drugs to Isle of Wight – Throughout the month of July, cancer drugs were flown from Portsmouth to a hospital on the Isle of Wight via drone.






