
GPhC Inspections: Common trends
GPhC inspection 2021 findings and the common trends split into the 5 principles.

GPhC inspection 2021 findings and the common trends split into the 5 principles.


The UK has now got out of the EU (BREXIT complete). As a WDA(H) you will need to transition from RP to RPi as advised by the MHRA.

Avicena a venture capitalist group has been busy seeing the acquisition of Dudley Taylor Pharmacy group (57 branches across England and Wales) and Shepherds pharmacy group
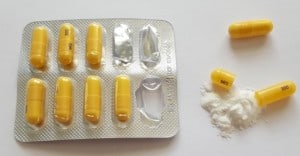
Pregabalin has been removed from the Northern Ireland (NI) Formulary for neuropathic pain due to an increased number of deaths.

A recent study suggests that three periods of dynamic brain changes that maybe particularly sensitive to the nuerotoxic effects of alcohol…