New packaging deadlines for UK products
All UK MA holder have to register for a new Great Britain Marketing Authorisation to convert their previous EU wide MA. They will get new Great Britain MA number therefore the packaging have to be changed…
All UK MA holder have to register for a new Great Britain Marketing Authorisation to convert their previous EU wide MA. They will get new Great Britain MA number therefore the packaging have to be changed…
It has been debated over the last three years whether a pharmacist can work remotely in crisis situations and it has arisen again considering the pressures we are facing during the Covid-19 outbreak…
In the past, the MHRA has told a pharmaceutical wholesaler to find a new Responsible Person when they did not know the correct definition for “GDP” in their MHRA inspection. The RP used the “ Gross Domestic Product “ definition to his peril…
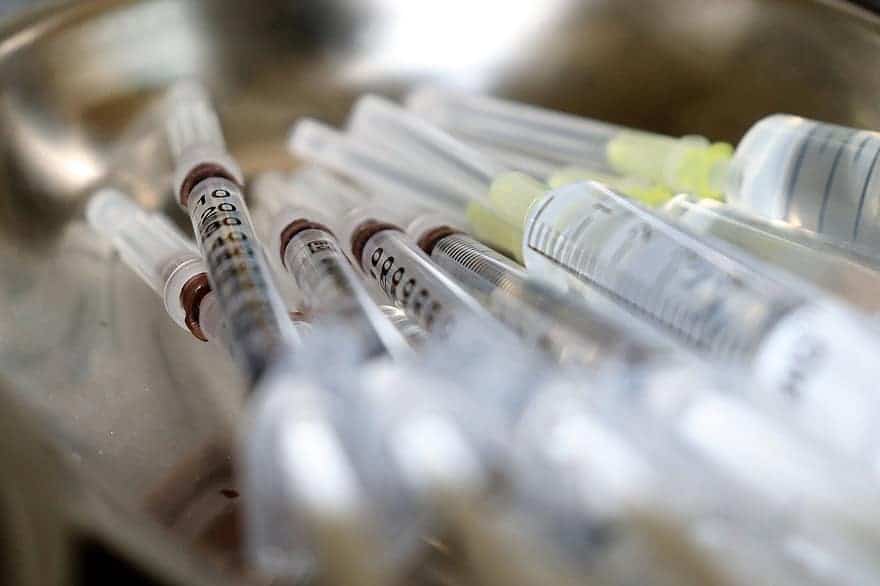
Vaccines are critical to public health as they prevent disease and curb community transmission…
Covid-19 will become synonymous with ‘working from home’ and has caused many businesses to re-consider how they operate day to day. So given that the required technology has been around for some years, why has it taken Covid-19 to prompt this re-think…
I am not a fan of business jargon, but I came across this term some years ago, not sure where. Whilst I am not even sure of the original meaning it has stuck with me as a possible name for an area of business analysis that experience would show, many organisations ignore or at least don’t do very well…
Export from EU to the UK.
Remember, at 11 pm on 31st December 2020, the UK will leave the EU Single Market and Customs Union…
When did you last mapped your warehouse?
EU goods imported into Great Britain after the Transition Period has ended will be subject to new UK Border controls which will be introduced in stages. This should give businesses affected by coronavirus a little more time to prepare. New border control post will be built and monies have been set aside to help fund the changes required and stakeholders…