Blog
Temperature controlled transport
Temperature controlled transport, are you transporting Pharmaceuticals correctly? This is a hot topic right now, especially with the unprecedented weather we have begun to experience lately.
Covid Booster Supply Issues
Covid Booster Supply Issues – Many pharmacies have reported that they are only receiving ‘a fraction’ of the Covid booster vaccines in their order deliveries. Despite placing orders for the vaccines, one pharmacy leader has spoken out and claimed they aren’t receiving their allocated deliveries of the boosters and are worried they will not have enough supply and may have to turn patients away.
Four things the GPhC is committed to
Back in June this year, GPhC faced so much criticism for the delays, IT disruptions and stress caused for many trainee pharmacists sitting their exam.
Public back pharmacy outcry
A survey commissioned by the National Pharmacy Association (NPA) found that two thirds of the those who took part found it unfair that pharmacies in England have not seen their budgets increase for at least 8 years – many are struggling with the huge increase in costs of running a pharmacy.
From Doncaster Pharmaceuticals
From Doncaster Pharmaceuticals Group Limited, to Doncaster Pharma Limited, BModesto Group, are relaunching and rebranding Doncaster Pharmaceuticals Group Limited (DPGL) after a recent partial buyout.
Fake online Pharmacies equal fake meds
Fake online pharmacies equal fake meds, and there are still online pharmacies who are operating illegally and suppling fake/counterfeit medications to the public for a fee.
Pilot scheme cycling and walking prescription
A new pilot scheme will award 11 local authorities’ part of a £12.7 million sum to help them offer prescriptions for walking, cycling, and wheeling. The pilots are a commitment to the governments Gear Change Plan that was introduced in 2020. The funding will...
Orphan medicines
Orphan medicines are medicinal products that are intended to treat diseases which are so uncommon that it would be difficult to secure funding for drug development under usual marketing conditions.
Conditional marketing authorisations
Conditional Marketing Authorisations (CMAs) were introduced in Great Britain as a scheme by the MHRA in January 2021. The CMAs can be granted for new medicinal products in GB whose benefits outweigh the potential risks of the new medicinal product.
Do you supply to Northern Ireland?
Do you supply to customers in Northern Ireland – If you do, then you will be aware of a range of issues following Brexit and that we are still some way away from a long term agreement. However, we wanted to highlight a specific issue.
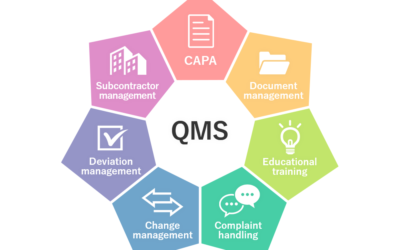
Robust Quality Management Systems
Robust Quality Management Systems (QMS) are the heart of our Quality Management. With the right process in place for your business model and the right people managing it, the Quality System will be robust, compliant, easy to navigate and a fantastic tool for trending.
Pressure on the NHS is set to increase
In an unprecedented move NHS bosses appeal to the Government to address rising costs before the winter causes a public health emergency.