PCL as a company has started to see that detectability has become a requirement when conducting Risk Assessments. There is more than one way to incorporate detectability in your risk assessments but below is a suggestion that could be used.
When conducting a risk assessment, I’m sure everybody has seen the following table or a version of, giving the risk a score for severity and likelihood and multiplying them to get a risk score in turn labelling that risk as High, Medium or Low.
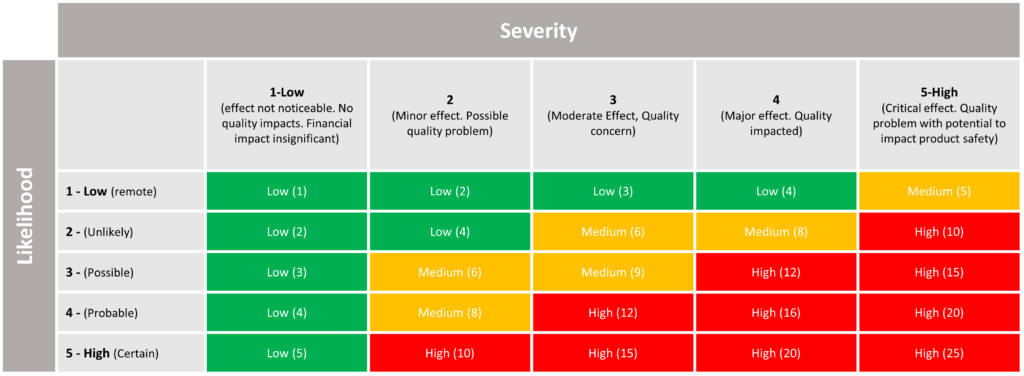
Table developed as per guidance taken from HSE “Risk Assessment & Management”.
The following table is a suggestion on how while still using the above table detectability can be incorporated into assessing a risk.
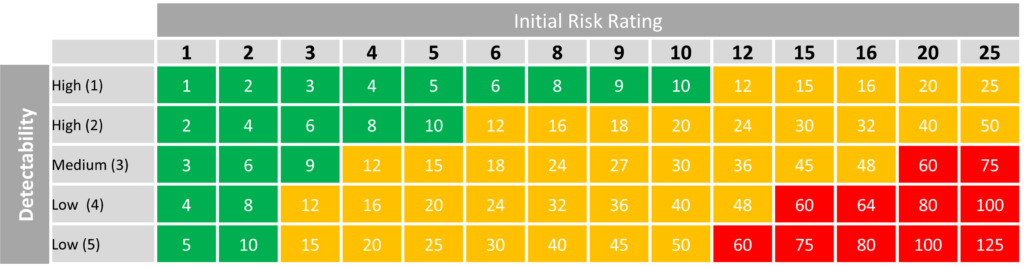
As with the severity x likelihood, Red is High Risk, Yellow is Medium Risk and Green is Low Risk. Following this suggestion, risk should be given an initial rating based on severity and likelihood, then taken into account detectability given an initial overall rating.
Once you have the initial overall rating the measures to be put in place should be documented distinguishing between control and detectability measures for example, assessing the risk of temperature excursions while the product is in storage then putting a procedure in place and ensuring all relevant personnel are trained and their competency tested would be a control measure. Installing data loggers/temperature monitoring systems with set alarms that alert all relevant personnel in the event of an excursion would be a detectability measure.
The risk then should be re-rated using both tables above to give a residual risk rating.
Remember every aspect of your business that even has a remote possibility of affecting product and/or patient safety must be Risk Assessed and all Risk Assessments should be reviewed periodically.
