
by Claire Dugdale | Oct 7, 2025 | Blog, CQC
PRN (pro re nata) medicines, also known as ‘when required’ medicines, are medicines with a ‘when required’ dose that can treat many different conditions (examples include nausea and vomiting, pain, indigestion, constipation, anxiety and insomnia).

by Claire Dugdale | Oct 1, 2025 | Blog, Pharmacy
Several regulatory changes are due to come into effect today (1st October 2025), having been included in the amendment regulations laid before Parliament last week (The National Health Service (Pharmaceutical and Local Pharmaceutical Services) (Miscellaneous Amendments) Regulations 2025).

by Claire Dugdale | Oct 1, 2025 | Blog, Distance Selling Pharmacy, Pharmacy
DSP (Distance selling premises) pharmacies will no longer be able to provide Directed services (Advanced, National Enhanced and Enhanced Services) face-to-face with patients at the pharmacy premises from today (1st October 2025).
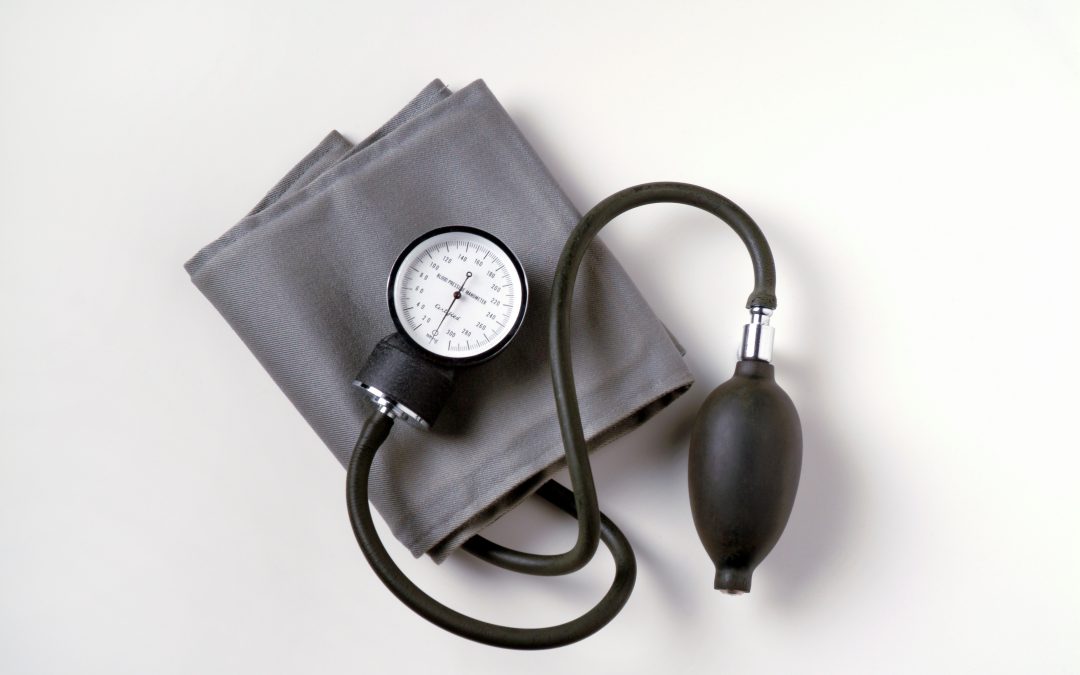
by Claire Dugdale | Sep 30, 2025 | Blog, NHS
DHSC, NHS England and Community Pharmacy England have agreed that the requirement to provide one Ambulatory Blood Pressure Monitoring (ABPM) consultation per month as part of the ‘bundling’ requirements to achieve Pharmacy First fixed monthly payment will not go ahead from 1st October 2025 as originally planned.

by Claire Dugdale | Sep 24, 2025 | Blog, NHS, Pharmacy
Is your pharmacy ready for the eligible adults being able to access an NHS flu vaccination from the 1st October 2025?

by Sally Finlayson | Sep 15, 2025 | Blog, Consultancy, MHRA, Pharmacy, Regulation, WDA(H)
Pharmacists need to check their Professional Indemnity Insurance to ensure that it covers them for the services they are providing, and to ensure that the level of cover is sufficient.






