
MHRA Green Guide – 2025 Update?
Pharmacists need to check their Professional Indemnity Insurance to ensure that it covers them for the services they are providing, and to ensure that the level of cover is sufficient.

Pharmacists need to check their Professional Indemnity Insurance to ensure that it covers them for the services they are providing, and to ensure that the level of cover is sufficient.

The pharmaceutical wholesale industry is a dynamic and highly regulated field, requiring businesses to adhere to stringent compliance standards. Pharmacy Consulting Ltd (PCL) provides comprehensive support to help wholesalers navigate these complexities, offering expert guidance in obtaining and maintaining Wholesale Distribution Authorizations (WDA) and ensuring adherence to Good Distribution Practice (GDP) guidelines.
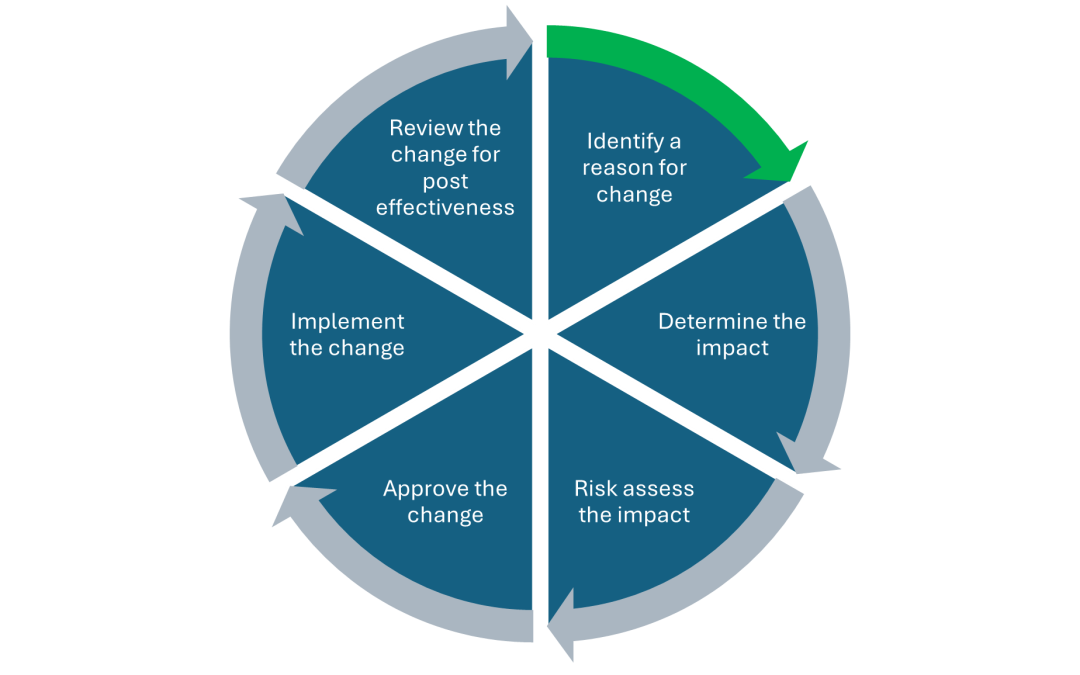
Management of change in your business should be a main cornerstone of any robust and functioning Quality Management System (QMS). The main advantages of having a strong change control process within your business include:

Self-Inspections are an important part of GDP and ensuring you keep your activities and licence compliant.
We are delighted to launch an updated Medical Device training and consultancy offering.

MHRA Guidance Note 6 – Notes for applicants and holders of a Wholesale Dealer’s Licence (WDA(H)) or Broker Registration was updated in November 2022 with some significant changes regarding Brexit.