
The Windsor Framework Part 1 (recap)
From the 1st of January 2025 the supply of medicines to Northern Ireland will be governed by the Windsor Framework, which will replace the Northern Ireland Protocol.

From the 1st of January 2025 the supply of medicines to Northern Ireland will be governed by the Windsor Framework, which will replace the Northern Ireland Protocol.
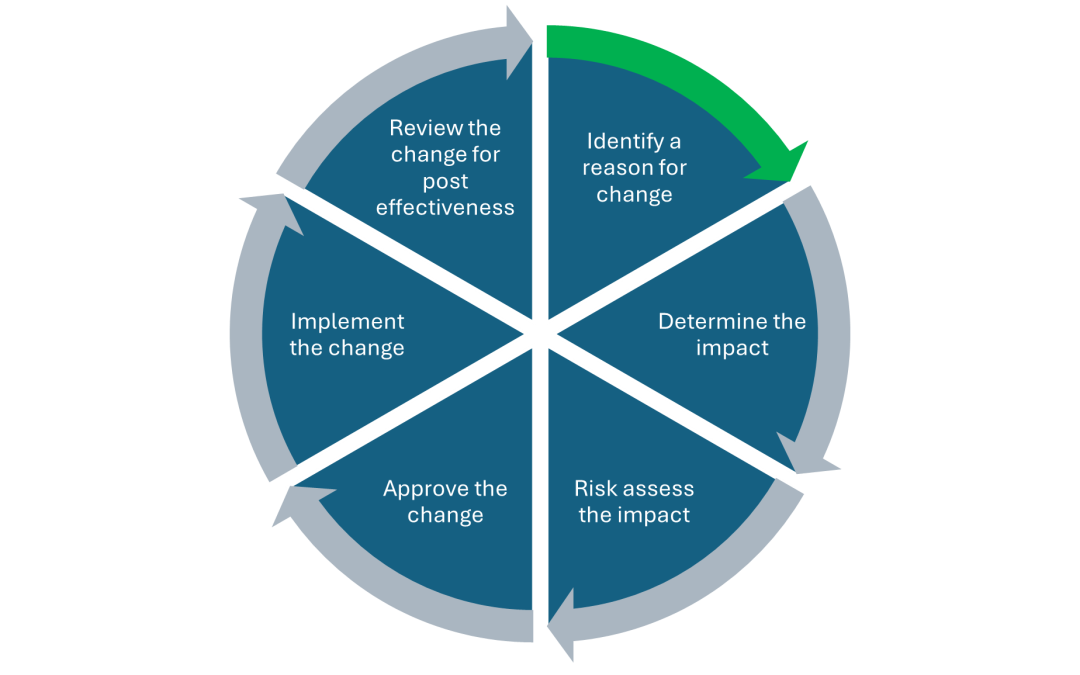
Management of change in your business should be a main cornerstone of any robust and functioning Quality Management System (QMS). The main advantages of having a strong change control process within your business include:

Most of us will know that it is illegal to promote prescription only medicines (POMs) to members of the public, however, it is always worth a reminder that you must be careful what you advertise.

Self-Inspections are an important part of GDP and ensuring you keep your activities and licence compliant.

Those companies manufacturing and handling medical devices should be aware that there are changes to the UK regulatory environment coming.

The MHRA have made the first release of their RegulatoryConnect service available which allows tracking of the submission status of product licence applications but will soon offer much more.