by Amelia Holmes | Jul 13, 2022 | Blog, Good Distribution Practice - GDP, MHRA, WDA(H)
An Introduced Product is an unlicensed medicine which can be imported from a country other than an approved country for import to be exported to a country other than an approved country for import, or if imported from a non-EEA country into Northern Ireland for export back to a country outside the EEA.

by Kimberley Peck | Jul 1, 2022 | Blog, GPhC
GPhC Inspections: Common trends 6 months into 2022, this blog takes a look at the common trends identified from inspections carried out this year.

by Chole Greenway | Jul 1, 2022 | Blog, MHRA, WDA(H)
For the distribution of medicines and healthcare products, a licence, authorisation or registration is required depending on the type of activity.

by Kimberley Peck | Jul 1, 2022 | Blog, CQC
Are you a Care Quality Commission (CQC) provider? Are you aware the training requirements for your staff members have changed?

by Sally Cox | Jun 27, 2022 | Blog, Training
You work in different ways to others, just the same as I do. However, for me (and maybe you) it just works when I learn face to face.

by Sally Cox | Jun 27, 2022 | Blog, GPhC
The Government recently announced that the emergency powers that were put in place by the Secretary of State for Health and Social Care to help with the response to the COVID-19 pandemic, will be removed later this year.
by Steven Hewison | Jun 27, 2022 | Blog, Medical Devices
Following the UK’s exit from the European Union (EU), the government identified a unique opportunity to improve how medical devices and in vitro diagnostic medical devices (IVDs) are regulated in the United Kingdom.
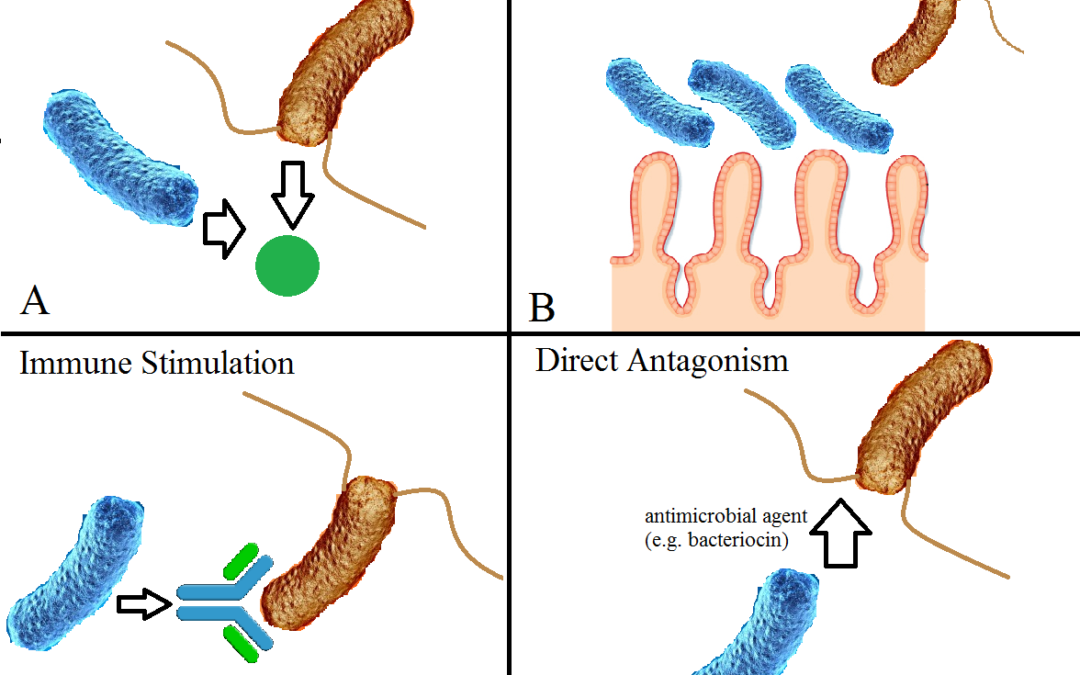
by Thomas Currie | Jun 24, 2022 | Blog
Probiotics are live bacteria that can colonise the gastrointestinal tract. Probiotic bacteria are often called ‘healthy bacteria’.
by Thomas Currie | Jun 24, 2022 | Blog
Antibiotic Dependence and how vaccines help, vaccines are a prophylactic treatment, meaning they must be administered before a viral or bacterial infection.
by Thomas Currie | Jun 24, 2022 | Blog, Covid 19
Antimicrobial surfaces – Work Smarter not Harder, As we slowly come out of the Covid-19 pandemic, it is hard to forget slogans such as ‘Hands, Face, Space’.










