
GPhC Standards app closure
The regulatory body for pharmacists, pharmacy technicians, and pharmacies in Great Britain will be discontinuing their ‘GPhC Standards App’ on 20th March 2022.

The regulatory body for pharmacists, pharmacy technicians, and pharmacies in Great Britain will be discontinuing their ‘GPhC Standards App’ on 20th March 2022.

Unlicensed Medicines (Specials) can only be supplied under one of the following circumstances:

Historically, women have always had a substantial role to play in pharmacy, however, when the Royal Pharmaceutical Society was founded in Great Britain on 15th April 1841.

As part of our covid risk assessment and the lifting of the restrictions in the UK we are delighted to be able to once again offer our popular ‘Face to Face’ ‘Cogent Gold Standard Responsible Person Training Course’.

Unlicensed Specials – Medicines made by manufacturers under a “Specials” licence.
Prescribing and Dispensing – Rural GP practices can act as Dispensing Doctors which means they can perform both the Prescribing and Dispensing of medication if it would be difficult for their patients to access a local pharmacy.
Advanced Therapy Medicinal Products (ATMPs) are medicines based on genes, tissues or cells for human use. ATMPs offer an innovative perspective for the treatment of disease and injury.

Licences for Medicines Wholesale – Do you know what is required by you as a pharmaceutical wholesaler to fulfil legal requirements when working with medicines? Read on to learn the basics of why you need a WDA and where to find further information to accelerate your learning.

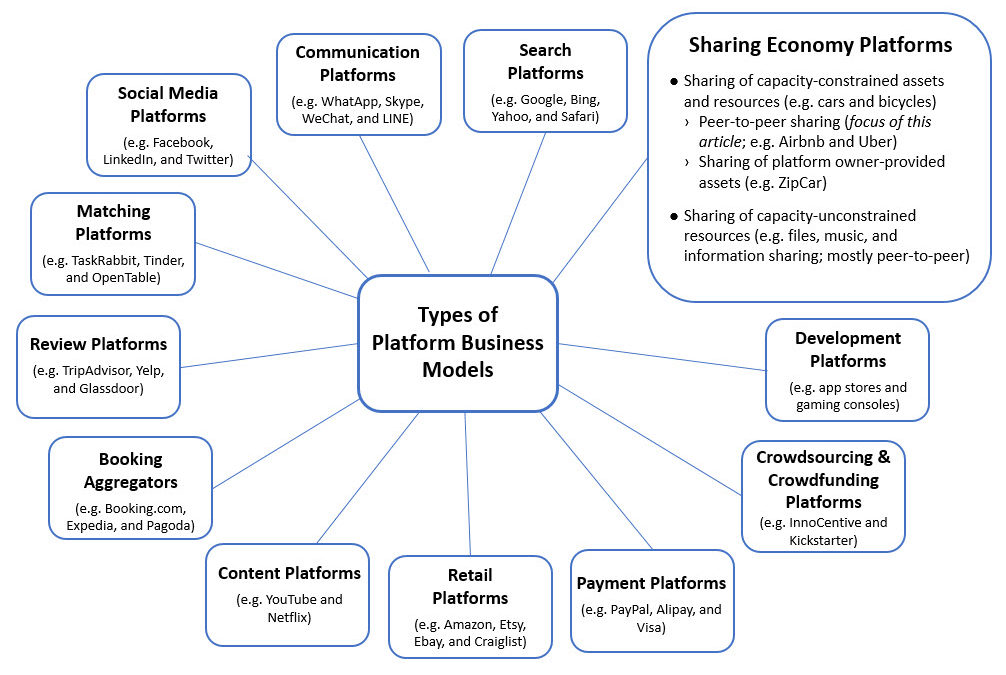
New Business Models; Most of us use one of the online food ordering and local delivery services. For many of us they have been an absolute godsend during the pandemic, meaning we can still get a regular fix of our favourite takeaways